
Unique selling points
- High removal percentage from water of recalcitrant organic compounds (such as emerging pollutants or priority substances) (higher than 95%) and microorganisms (higher than 99.9%).
- Most cost-effective treatment than other AOPs, such as UV/O3.
Description of the technology
H2O2 photolysis (UV + H2O2) is and Advanced Oxidation Process (AOP) that is based on hydrogen peroxide disproportion to hydroxyl radical (●OH). Hydroxyl radicals are one of the most powerful oxidant species, as they have the capacity to non-selectively degrade or also mineralize different types of organic matter, and this technology is the simplest way to obtain them.
The high disinfection capacity of this technology lies in the fact that UV radiation can work simultaneously photolyzing peroxide and acting as a disinfectant, which leads to the production of highly reactive hydroxyl radical species.
Thanks to the synergetic effect of hydrogen peroxide, hydroxyl radicals and UV-C light can produce microorganism damage and therefore their subsequent inactivation (Black & Veatch Corporation, 2009).
However, it should be highlighted that the efficiency of the ●OH radical production depends on hydrogen peroxide capability to absorb UV radiation (which is lower than the molar absorptivity of ozone) and also on the physical and chemical characteristics of the fluid that will be submitted to the H2O2 photolysis (Mierzwa et al., 2018).
In the NextGen project, H2O2 photolysis is applied to disinfect and to remove trace organic compounds (TrOC) such as the plant protection products (PPPs) before all the horticulture streams were reused in the greenhouses (according to the Netherlands legislation that obliges to the zero emission).
The capacity of the process in NextGen is 1-2 m3/h.
Flow scheme of the technology
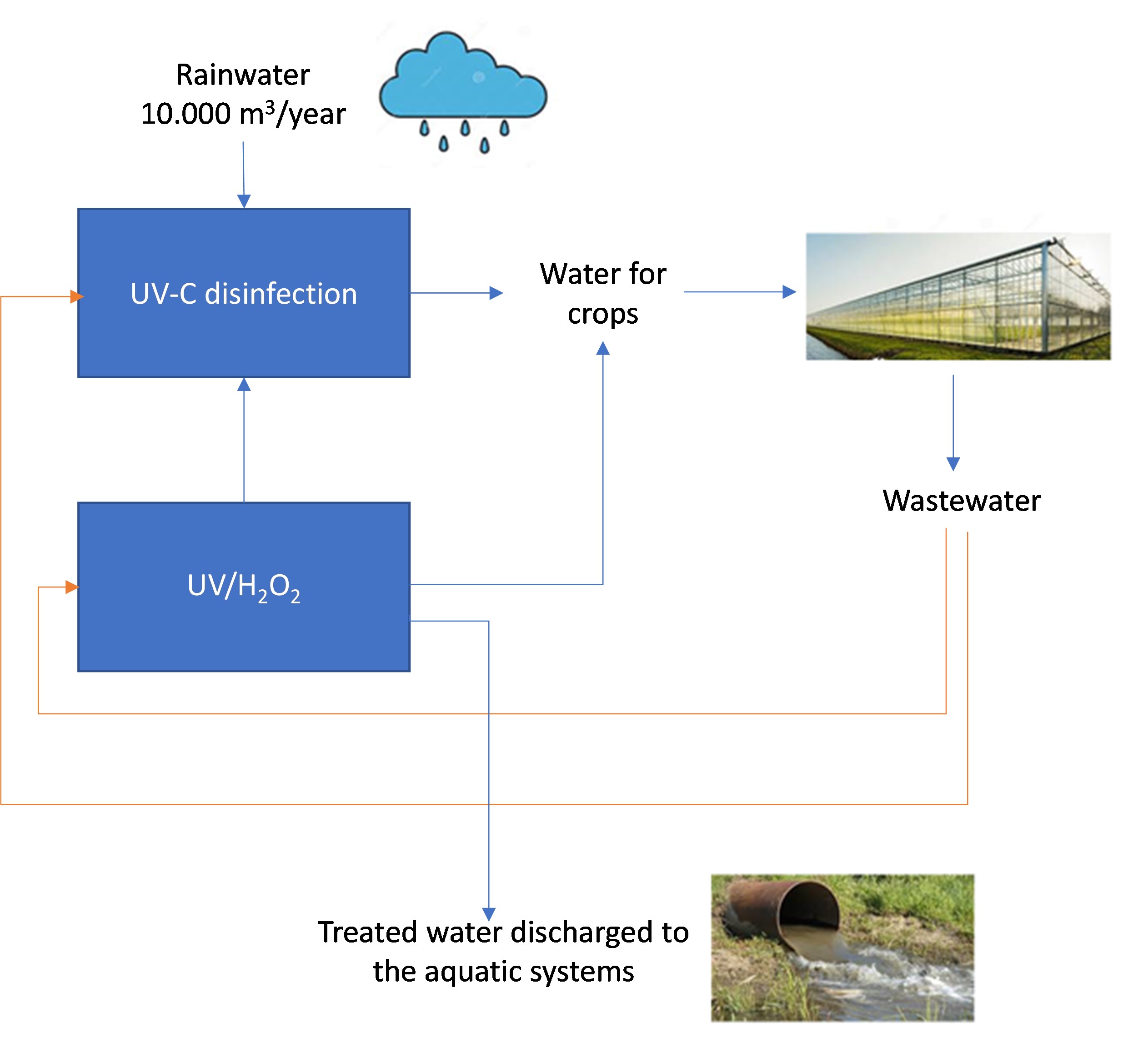
Figure 1. Scheme of the horticulture circular water scheme where UV/H2O2 photolysis technology is applied (Westland case study, Netherlands).
Pictures
Synergetic effects and motivation for the implementation of the technology
- Reduction of the emerging/priority pollutants that reach aquatic systems.
By removing plant protection products (PPPs) or other emerging/priority substances from the horticulture streams and by recycling part of them in the greenhouses, NextGen will contribute to avoiding that certain organic substances reach aquatic systems thus preventing certain effects on the fauna related to endocrine disrupting process as well as protecting the flora.
- Reduction of the drinking water consumption destined to irrigation.
Hydrogen peroxide photolysis will remove organic pollutants and disinfect the horticulture wastewater. Therefore, it will allow to produce regenerated water to be used for irrigation purposed in the greenhouses to focus on zero PPPS discharge legislation. This will contribute to reduce the quantity of drinking water consumed for this application, therefore improving the availability of this resource.
Technology requirements and operating conditions
A mesh filter must be incorporated at the beginning of the treatment to ensure that any coarse solid reaches the AOP treatment. Turbidity values in the influent higher than 20 NTUs indicate that a pre-treatment is needed. Otherwise, the H2O2 photolysis will destroy part of the suspended solids and redissolve them, implying an increase on dissolved organic content of the water.
The following table summarize the most appropriated values of several parameters.
Table 1. Required specifications for the H2O2 photolysis system.
|
Parameter |
Units |
Min |
Max |
Reference |
|
Turbidity |
NTU |
0 |
20 |
The presence of the suspended solids significantly affects the effluent optical properties, mainly the UV radiation transmission (Mierzwa et al., 2018). |
|
Temperature (at atmospheric pressure) |
ºC |
5 |
35 |
The H2O2 decomposition increases with the temperature. At 20 ºC is around 4%; whereas at 30ºC increase at 11%. At 50ºC its decompisition is around 80% (Yazizi and Deveci, 2010). |
|
pH |
- |
0 |
14 |
Hydroperoxide anion, HO2-, generated at basic conditions, has a significantly higher molar absorption coefficient than H2O2 itself (240 L/mol per cm at 254 nm (Oppenländer, 2002). |
|
UV wavelength |
nm |
200 (Ꜫ = 180 L/mol) |
562 calculated energy for the homolytic cleavage of the central O-O |
UV absorption by H2O2 increases as the wavelength decreases (at 200nm, Ꜫ = 180 L/mol; at 300 nm Ꜫ decreases to 0.88L/mol (Cataldo, 2014; Lide, 2006-2007). |
Key performance indicators
The specific KPIs for the UV/H2O2 system during NextGen project is detailed in the following table.
Table 2. KPIs for the H2O2 photolysis system.
|
Parameter |
Units |
Min |
Max |
Reference |
|
Degradation of specific TrOCs |
% |
< 10 |
> 90 |
Cuerda-Correa et al., 2020 |
|
Global removal yield of organic matter |
% of DOC removed |
< 10 |
> 90 |
Highly dependant on the matrix and conditions. (Vilhunen et al., 2010). |
Links to related topics and similar reference projects
|
H2O2 photolysis |
Reference |
|
NextGen |
Publications
- Cataldo, F., Hydrogen peroxide photolysis with different UV light sources including a new UV-Led light source., 2014
- Cuerda-Correa, E., Alexandre-Franco, M. F and Fernández-González, C., Advanced Oxidation Processes for the Removal of Antibiotics from Water. An Overview., 2020
- Lide, D.R., CRC Handbook of Chemistry and Physics. A Ready-Reference Book of Chemical and Physical Data, 2005
- Mierzwa, J.C., Rodrigues, R. and Teixeira, A. C.S.C., Chapter 2 - UV-Hydrogen Peroxide Processes. In: Suresh C. A. and Rakshit A. (Ed). Advanced Oxidation Processes for Waste Water Treatment., 2018
- Oppenländer, T., Photochemical Purification of Water and Air: Advanced Oxidation Processes (AOPs): Principles, Reaction Mechanisms, Reactor Concepts., 2002
- Rosario-Ortiz, F.L., Wert, E.C., Snyder, S.A., valuation of UV/H2O2 treatment for the oxidation of pharmaceuticals in wastewater, 2010
- Vilhunen, S., Vilve, M., Vepsalainen, M. and Sillanpaa M., Removal of organic matter from a variety of water matrices by UV photolysis and UV/H2O2 method., 2010
- Yazizi, E. Y. and Deveci, H., Factors Affecting Decomposition of Hydrogen Peroxide., 2010

